Rita Food & Drink Co.,Ltd announces a streamlined OEM/ODM beverage program engineered for rapid market entry in the GCC and EU. The offer combines HALAL conformity for Gulf markets, US FDA–ready documentation for global buyers, EU labeling support, and transparent lead times & MOQs—so you can move from brief to shelves with confidence.
Key takeaways
- Compliance-first: HALAL route aligned with GSO 2055-1 and national schemes (e.g., Saudi Halal Center, UAE Halal National Mark); EU labels per Reg. (EU) 1169/2011 and traceability under Reg. (EC) 178/2002.
- US FDA readiness: Support with food facility registration (21 CFR 1.225), FSVP documentation for your U.S. importer, Juice HACCP (21 CFR 120), and—if applicable—LACF/AF process filings (FCE/SID).
- Lead time (ex‑factory): typically 25–40 working days after formula & artwork approval (includes production scheduling and QA release).
- MOQs (guidance):
- 330 ml cans: from 1 × 20’ container per SKU (≈ 36,000–48,000 units, pack-out dependent).
- 250–500 ml PET: from 10,000–20,000 bottles per SKU.
- Glass bottles: from 5,000–15,000 bottles per SKU.
- Labeling languages: Arabic (GCC) + target EU Member State language(s) as required; artwork templates provided.
- Document pack: HALAL certificate (where applicable), specs & COA, nutritional data, ingredient list & allergens, batch traceability, shipping docs.
Why “HALAL + US FDA Ready” matters for GCC/EU buyers
GCC retail and import clearance commonly require HALAL compliance built on GSO 2055-1 “Halal Food — General Requirements.” National authorities such as the Saudi Food & Drug Authority (SFDA) Halal Center and the UAE’s MOIAT operate recognition and national mark programs that rely on GSO requirements. Working within these schemes ensures your SKUs are accepted at ports and on shelves.
For EU, your label must meet Reg. (EU) 1169/2011 (mandatory particulars like ingredient list, allergens, and nutrition declaration) and your supply chain must support traceability “one step back, one step forward” under Reg. (EC) 178/2002—Rita’s batch coding, retained samples, and documentation systems are designed around that principle.
Although US FDA registration is not an EU import requirement, buyers often request FDA-grade documentation because it signals robust controls. For U.S.-direct shipments, Rita supports facility registration (21 CFR 1.225), importer FSVP (21 CFR Part 1 Subpart L) documentation, Juice HACCP (21 CFR 120), and—if your product qualifies—LACF/AF filing (FCE/SID) per 21 CFR 113/114.
The compliance roadmap (what we do for you)
- Product brief & formula screening (Days 1–5)
- Confirm target markets (GCC/EU/US), ingredient restrictions, sweetener/preservative choices, and shelf‑life
- Early check for HALAL eligibility (alcohol-free flavor carriers, processing aids, etc.).
- Regulatory scoping (Days 3–7)
- GCC HALAL path selection: either within an existing scope or via audit with a recognized HALAL certification body (e.g., SFDA Halal Center–listed).
- EU: map label particulars per (EU) 1169/2011; plan allergens, nutrition table, net quantity, durability date, operator address, and language.
- US (if needed): confirm facility registration status and whether product falls under Juice HACCP and/or LACF/AF.
- Samples, artwork & claims check (Days 5–14)
- Lab samples for taste, brix, acidity, stability.
- Artwork against Arabic requirements for GCC and the EU Member State language(s).
- Certification & listings (variable)
- If facility/formula are already covered by a recognized HALAL scope, certificate issuance is usually administrative. If not, we coordinate the audit cadence with your chosen body.
- If U.S. shipment is in scope: we support FSVP documentation for your importer; where applicable, we coordinate process filing (FCE/SID) with a qualified process authority.
- Production, QA release & logistics (Days 15–40)
- Commercial run, microbiological tests, COA issuance, export docs, and container loading (FOB Cat Lai, or CIF/CFR on request).
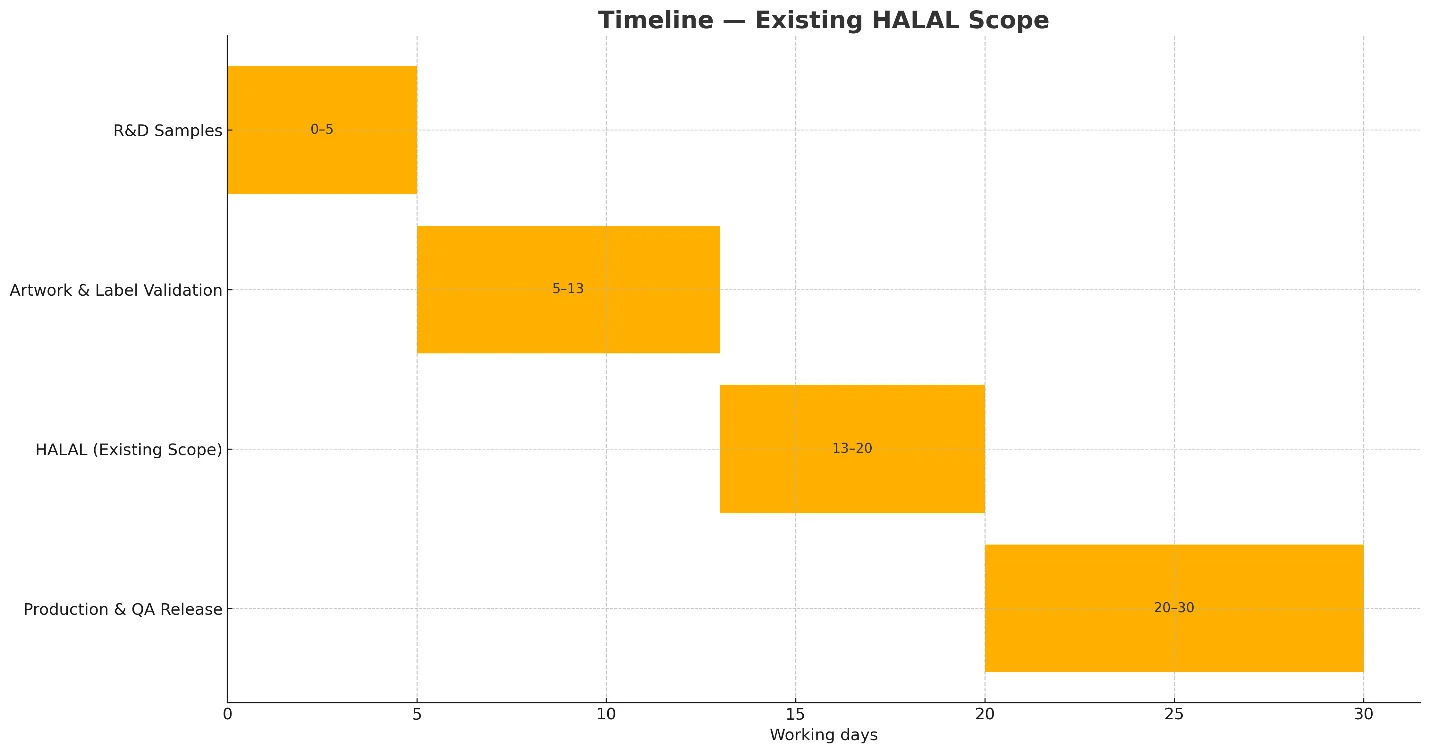
Lead times (typical)
- R&D samples: 3–7 business days after brief.
- Artwork & label validation: 5–10 business days.
- Certification window:
- HALAL in existing scope: administrative issuance (timing depends on body).
- New scope / audit required: generally 2–6 weeks depending on auditor availability.
- Production & QA release: 7–14 business days from materials-in.
- Total ex‑factory: usually 25–40 working days after formula & artwork approvals (shipping transit to Jebel Ali, Dammam, Rotterdam, etc., varies by carrier and season).
Note: timelines reflect typical Rita schedules; certification bodies’ calendars, packaging procurement, and peak seasons can affect exact dates.
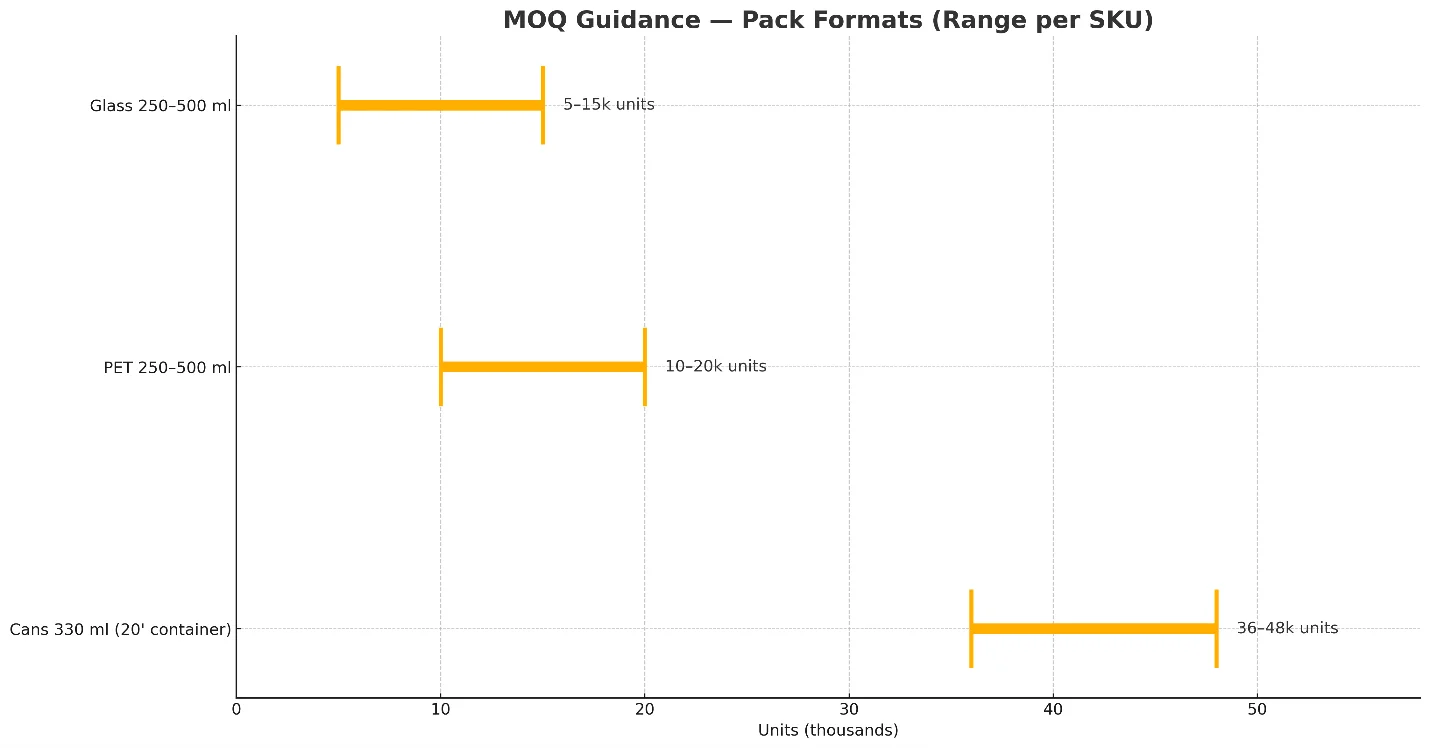
MOQ & pack formats (guidance)
- 330 ml sleek cans / 250–330 ml std. cans
- From 1 × 20’ container per SKU; depending on case count and palletization this equates to roughly 36,000–48,000 cans.
- PET (250–500 ml)
- From 10,000–20,000 bottles per SKU (label‑wrapped or sleeved).
- Glass (250–500 ml)
- From 5,000–15,000 bottles per SKU.
- Pilot runs
- Lower MOQs may be available using stock molds, neutral caps, and simplified artwork—ask for current slotting.
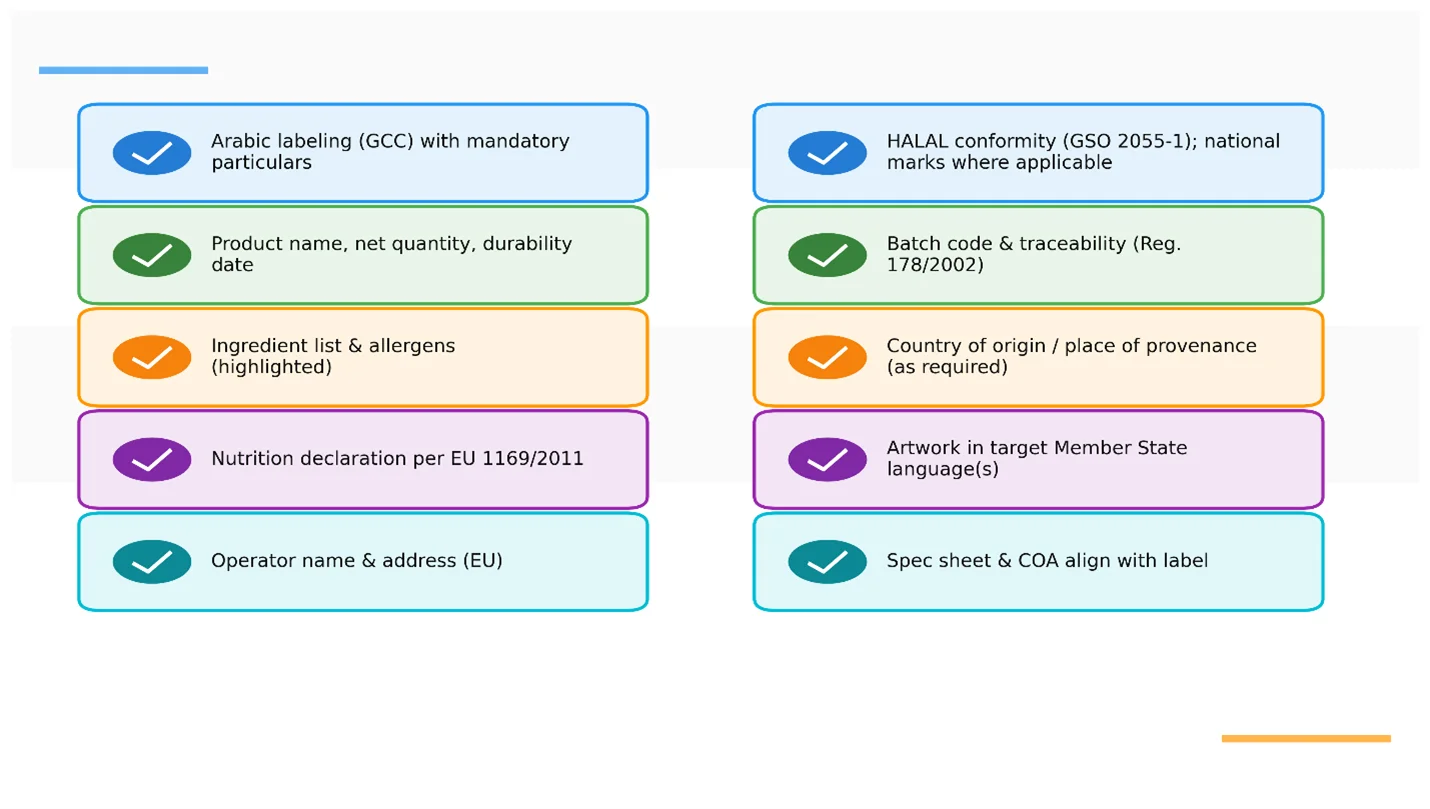
Labeling & languages (GCC/EU quick guide)
- GCC: Arabic on prepacked foods is required under GSO 9; artwork must reflect mandatory particulars (name, ingredients, COO, shelf life, etc.). We provide Arabic panel templates and translation support.
- EU: comply with Reg. (EU) 1169/2011 (e.g., allergens, nutrition declaration). Language is the official language(s) of the Member State of sale; we localize accordingly.
- Traceability: Rita maintains batch records to meet the “one step back—one step forward” rule under Reg. (EC) 178/2002.
What you receive (documentation pack)
- HALAL certificate (for GCC shipments) and/or UAE Halal National Mark dossier where applicable.
- Specs & COA, nutritional facts, ingredient list & allergens, MSDS (if needed).
- Batch traceability records and retained sample reference.
- Export documents: commercial invoice, packing list, certificate of origin; health/sanitary cert where required.
Why Rita
- End-to-end OEM/ODM: formula development, packaging sourcing, artwork, compliance, and global logistics.
- Regulatory fluency across GCC/EU/US frameworks with practical, buyer-facing documentation.
- Scalable capacity with competitive MOQs and stable lead times.
Suggested SEO keywords
OEM beverage, private label drinks, HALAL certification, US FDA, GCC market, EU labeling, FSVP, Juice HACCP, beverage OEM Vietnam, ODM drinks, lead time, MOQ, Arabic labeling, GSO 2055-1, Regulation (EU) 1169/2011, Regulation (EC) 178/2002, FCE/SID, low-acid canned foods, Rita Food & Drink Co.,Ltd.
FAQs
1) Do I need HALAL certification for all GCC countries?
For mainstream retail and food service in the GCC, HALAL conformity aligned with GSO 2055‑1 is the norm. In Saudi Arabia, HALAL certificates must be issued by bodies recognized by the SFDA Halal Center. In the UAE, products may carry the UAE Halal National Mark under MOIAT’s scheme. Rita coordinates the correct pathway for your SKU.
2) Is US FDA registration required to sell in the EU?
No. EU has its own legal framework. US FDA facility registration (21 CFR 1.225) is needed for foods destined for the United States. For EU, labels must meet Reg. (EU) 1169/2011 and supply chains must meet Reg. (EC) 178/2002 traceability.
3) Who handles FSVP for U.S. shipments?
Your U.S. importer of record must operate an FSVP program (21 CFR Part 1, Subpart L). Rita supplies the technical documentation (hazard analysis inputs, supplier assurance, process validations) your importer needs.
4) My drink is shelf‑stable in cans—do we need special filings?
If your formula is an acidified or low‑acid canned food, process filings (FCE/SID) and thermal process controls under 21 CFR 113/114 may apply. Rita can coordinate with a process authority to determine applicability and file as needed.
5) What languages must appear on the label?
- GCC: Arabic is required on prepacked products.
- EU: the official language(s) of the Member State where the product is sold. We localize labels accordingly.
6) What are Rita’s typical MOQs?
As guidance: 1×20’ container per SKU for cans; 10,000–20,000 for PET; 5,000–15,000 for glass. For pilot launches, talk to us about stock packaging to reduce MOQs.
7) How long does HALAL certification take?
If your SKU fits an existing certified scope, issuance can be fast. A new scope plus audit typically runs 2–6 weeks, dependent on the certification body’s schedule and document readiness (range for planning; exact timing varies).
8) What documents will customs/retailers expect?
HALAL certificate (for GCC), COA, specs, EU 1169/2011-compliant label proofs, traceability records under 178/2002, and standard export documents.
Ready to brief your next SKU?
Send your flavor, pack size, target markets, and preferred incoterms—Rita’s OEM team will return a tailored compliance and production plan with current lead time & MOQ for your exact configuration.


